Which Is True About the Dissolving Process in Water?
The dissolving process in water is a fundamental concept in chemistry and plays a crucial role in various natural and industrial processes. Understanding how substances dissolve in water is essential for scientists and engineers in fields such as pharmaceuticals, environmental science, and materials science. In this article, we will explore the true nature of the dissolving process in water and delve into its underlying principles.
1. Definition of Dissolving:
Dissolving refers to the process in which a solute (a substance that is being dissolved) disperses uniformly throughout a solvent (the substance in which the solute dissolves).
Water is often referred to as the universal solvent due to its ability to dissolve a wide range of substances.
2. Factors Affecting Dissolving:
Several factors influence the dissolving process in water, including:
a. Temperature:
Generally, an increase in temperature accelerates the dissolving process.
This is because higher temperatures increase the kinetic energy of both the solvent and solute particles, causing more collisions and facilitating the breakdown of intermolecular forces.
b. Surface Area:
The surface area of the solute also impacts the dissolving process. A larger surface area provides more contact points between the solute and the solvent, leading to a faster dissolution rate.
c. Agitation:
Agitating or stirring the solution enhances the dissolving process by increasing the contact between the solute and solvent. Stirring promotes the movement of solute particles away from the solid surface, facilitating their interaction with the solvent.
which is true about the dissolving process in water
3. Solubility:
Solubility refers to the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature and pressure. The solubility of different substances varies greatly.
Some substances, like sugar, have high solubility in water, while others, such as oil, have low solubility.
4. Polar and Nonpolar Substances:
Water is a polar molecule, meaning it has a partial positive charge on one end (hydrogen) and a partial negative charge on the other end (oxygen).
Polar substances tend to dissolve in water because the water molecules can surround and interact with the solute particles, breaking their intermolecular forces.
In contrast, nonpolar substances, like oil, do not dissolve easily in water.
The polar nature of water creates strong intermolecular forces within the water molecules, making it difficult for nonpolar solute particles to break these forces and mix with water.
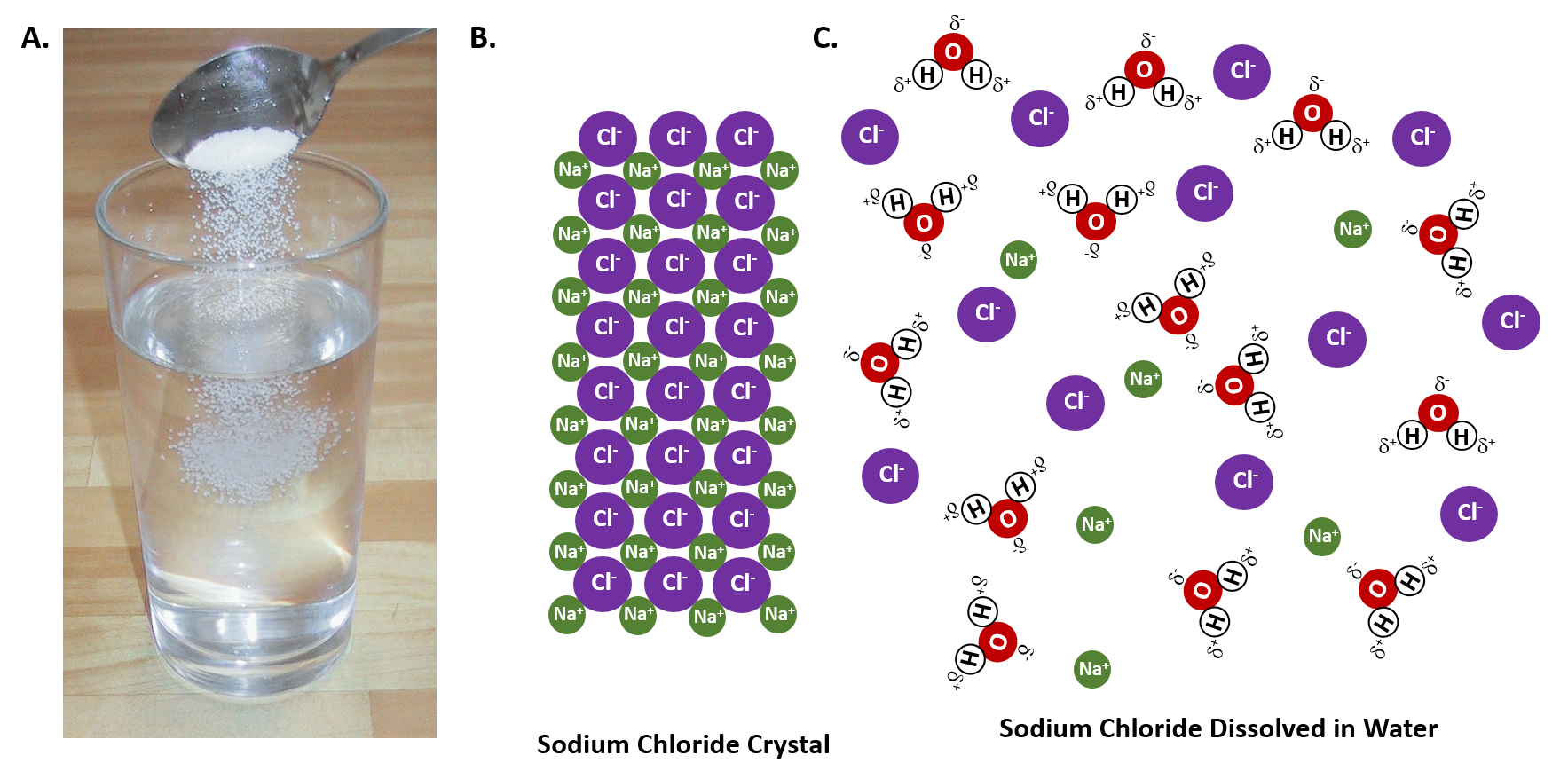
which is true about the dissolving process in water
5. Dissociation and Ionization:
Some substances, known as electrolytes, dissolve in water and undergo dissociation or ionization. Dissociation involves the separation of a compound into its constituent ions, while ionization refers to the formation of ions from neutral molecules.
These ions enable the solution to conduct electricity, a characteristic of electrolytes.

which is true about the dissolving process in water
6. Concentration:
The concentration of a dissolved substance in water is determined by the amount of solute present in a given volume of solvent. Concentration can be expressed in various ways, such as mass/volume percent, molarity, or parts per million.
The concentration affects the properties and behavior of the solution.
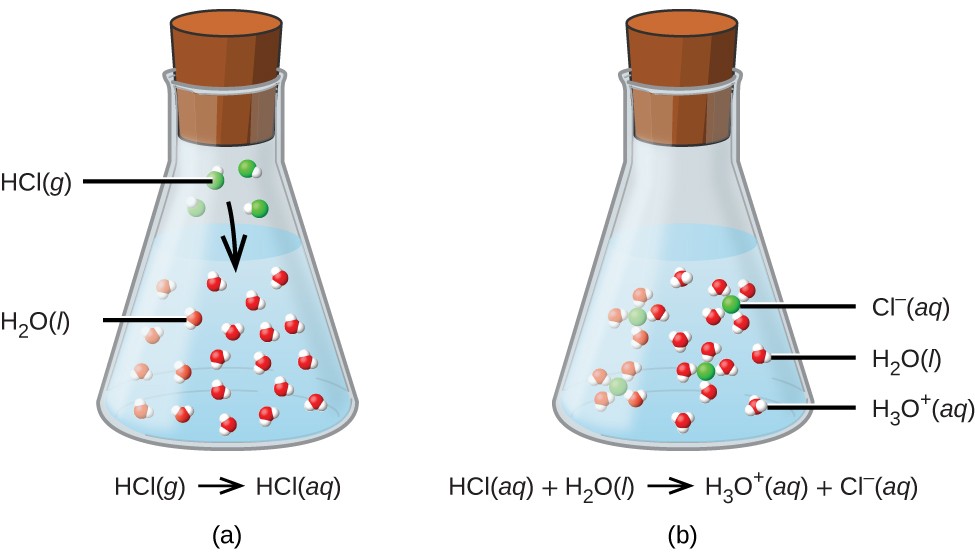
which is true about the dissolving process in water
7. Conclusion:
In conclusion, the dissolving process in water is influenced by various factors such as temperature, surface area, and agitation. Solubility, polarity, and the presence of electrolytes also play crucial roles.
Understanding the true nature of the dissolving process allows scientists and engineers to design and optimize processes in numerous fields.
By unraveling the mysteries of dissolution, we can further explore the remarkable properties and applications of water, the universal solvent.